A significant challenge faced by surgeons, physicians, nurses, and other healthcare professionals globally is achieving wound healing in various clinical settings. Regardless of etiology, the process of achieving optimal wound healing is governed by similar factors. This article will consider the role of recombinant growth factors in facilitating effective wound healing.
Wound Healing: Basic Physiology
The body's wound healing process follows a series of well-defined stages including the following:
- Hemostasis
- Inflammation
- Proliferation/granulation
- Maturation/remodelling
Recombinant growth factors play a crucial role in each of these stages by shortening healing times, facilitating the production of viable tissue, and improving overall outcomes.
Hemostasis
Immediately following an injury to the human body, its first response is to try and stop bleeding. Blood vessels affected by the injury will constrict while platelets will aggregate at the wound site to form a clot to achieve hemostasis.
Inflammation
Once hemostasis is complete and the injured area has been secured, the blood vessels dilate, allowing crucial nutrients, cells, chemical mediators, and enzymes to reach the wound site. White blood cells (leucocytes) and inflammatory cytokine remain unregulated at this stage of wound healing. Additionally, the physical signs of inflammation (pain, swelling, redness, and heat) are evident at this stage.
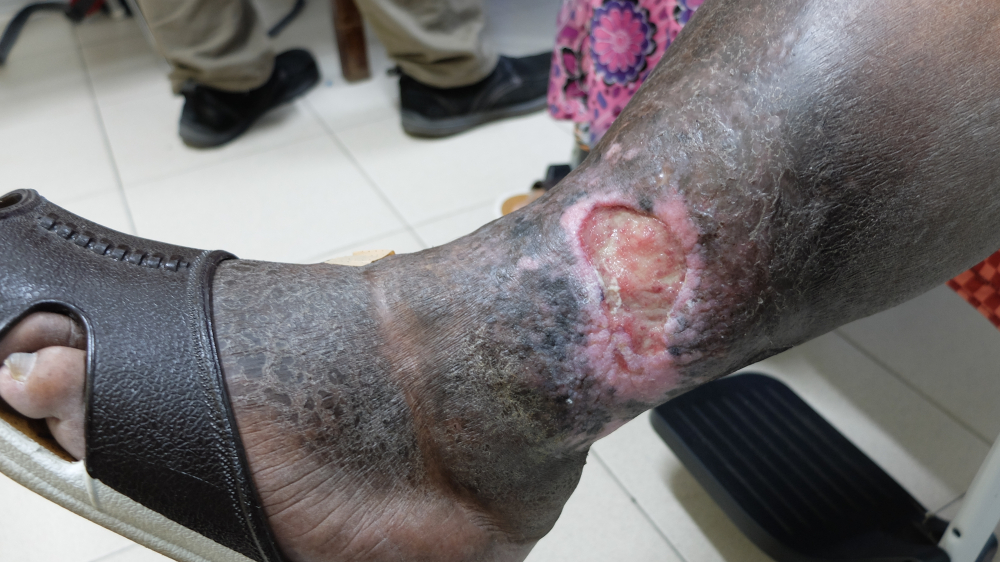
Proliferation/Granulation
The proliferation stage involves the closure of wounds with granulation tissue which forms the scaffold tissue for the completely healed wound. The factors critical to the deposition of healthy granulation tissue are the presence of sufficient oxygen and nutrients. Healthy granulation tissue appears pink while a darker color might indicate nutrient or oxygen deficit leading to poor wound healing. In addition to granulation tissue, the body also recruits fibroblasts derived from the damaged mesenchymal cells to further improve the strength and integrity of the healing wound.
Maturation
This is the last stage in a wound healing process and it also takes the longest amount of time to complete. Maturation commences when wound closure is complete and includes dermal remodeling activities to improve the strength of the tissue overlying the healed wound site. It is important to note that even when completely healed, the new tissue retains only about 80 percent of its original strength.
Role of Recombinant Growth Factors in Wound Healing
Human growth factors are endogenously derived signaling molecules typically proteins, that help regulate cellular responses during wound healing processes. These molecules can be secreted by various cell types including fibroblasts, epithelial cells, platelets, and leukocytes. Growth factors act in an endocrine, autocrine, or paracrine manner to activate the relevant cellular machinery needed for wound repair and healing. Recombinant growth factors, also known as exogenous growth factors, are signaling protein molecules synthesized outside human cells but possessing the ability to alter their physiological processes.
In recent times, scientists have gained a greater understanding of various molecules associated with wound healing. With improved molecular engineering and biological technology, the usefulness of various recombinant growth factors in wound healing is becoming more evident. Exogenous growth factors are currently being applied in controlled surgical scenarios to boost healing outcomes. Recombinant growth factors are fast becoming a routine adjunct in various cosmetic and corrective surgical procedures showing benefits in oral surgery, plastic surgery, orthopedic surgery, and burn surgery.
Examples of recombinant growth factors with potential for use in surgical and non-surgical (venous ulcers, diabetic foot ulcers) wound healing include:
- Platelet-derived growth factor (PDGF)
- Vascular endothelial growth factor (VEGF)
- Fibroblast growth factor (FBF)
- Epidermal growth factor (EGF)
- Keratinocyte growth factor (KGF)
- Granulocyte-macrophage colony-stimulating growth factor (GM-CSF)
- Transforming growth factor-beta (TGF-β)
Platelet-Derived Growth Factor
Human platelets are among the first cell types to get recruited to a fresh wound site. Apart from playing a crucial role in achieving hemostasis, platelets produce a platelet-derived growth factor. PDGF plays a key role in angiogenesis, cell growth, and cell division. It also functions as a chemoattractant for mesenchymal cells from which fibroblasts are derived. To date, PDGF is the only recombinant growth factor approved by the United States Food and Drug Administration for topical application in the treatment of diabetic foot ulcers.
Vascular Endothelial Growth Factor
The VEGF family of recombinant growth factors is a group of 6 signaling proteins namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor. VEGF-A is the most commonly studied member of the subset with a proven role in new blood vessel formation and the migration of their endothelial components. VEGF is usually secreted as an early response to tissue injury as well as tissue hypoxia. Clinical studies have shown VEGF-A to improve re-epithelialization in diabetic foot wounds while improving blood vessel formation and tissue perfusion.
Fibroblast Growth Factor
The fibroblast growth factor family comprises about 20 isoforms with FBF-2 also known as bFBF being the most studied member. The bFBF protein has been shown to improve the proliferation of epithelial and mesenchymal cells with a possible role in angiogenesis. Additional studies done with recombinant fibroblast growth factor have been shown the protein accelerates wound healing by stimulating extracellular matrix development, collagen deposition, and improving the tensile strength of healing wounds.
FBF-2 showed the best response among a group of growth factors used to improve wound healing in human patients with pressure ulcers. Also, bFBF has been used as an adjunct for treating burns and fractures. Fibroblast sprays are commercially available for topical application in skin ulcers.
Epidermal Growth Factor
Epidermal growth factor has been used to supplement the healing of skin grafts done for partial-thickness burns. EGF application reduced the duration of re-epithelialization compared to cases where no additional intervention was carried out.
A randomized controlled trial done using recombinant human epidermal growth factor yielded positive results providing evidence that topically administered EGF will stimulate chronic wound healing processes.
Transforming Growth Factor-Beta
TGF-β is a mitotic peptide whose main mechanism of action in wound healing is angiogenesis although some studies have suggested a possible role in wound re-epithelialization. However, limited data exist on the true significance of this signaling protein and further studies would be required to prove its efficacy in a clinical setting.
Limitations to Exogenous Growth Factor Use
While the mechanism of action of many growth factors is well understood, there is limited data on their effectiveness in managing acute, and chronic wounds. At present, a significant amount of what we know about recombinant growth factors comes from observing the wound healing responses of non-human test subjects with various exogenous proteins. A major challenge to gathering sufficient pre-clinical and clinical data from human test subjects is designing a safe and comprehensive test model.


.webp)

.avif)